Published on: October 28, 2019 | Updated on: August 29, 2024
Cell-based therapies offer new hope for treating illnesses that have otherwise had an unsuccessful treatment process. They have created new hope for finding relief from illnesses such as arthritis, spinal cord injuries, and even some types of cancer.
Before something that has so much potential, hits the market, it must be researched extensively to get the go-ahead from the agencies responsible for ensuring its safety.
What Are Stem Cells?
The term “stem cell” refers to cells that are the building blocks for human life. They are located throughout the body and are more concentrated in specific areas, such as bone marrow. The function of stem cells is to provide a basis for other cells to grow out of. These cells can grow into almost any organic cell in the human body, including:
- Muscle
- Bone
- Cartilage
- Tendons
- Skin cells
- Organ tissue
These are just a few examples of what stem cells can grow into. There are hundreds of possibilities for treatments using these cells that can be unlocked through research.
Stem cells used in research and clinical trials come from several parts of the body. These can include:
- Adipose (fat) tissue: these cells are most often taken from the patient that treatment is being performed on.
- Blood cells: stem cells can be extracted from blood cells using a simple blood draw and specialized processing techniques that concentrate the stem cells.
- Bone marrow: one of the more popular methods of extracting stem cells involves taking a sample from bone marrow. The procedure for this is quick and relatively painless.
- Umbilical cord tissue: these types of tissue are almost always received via the mother’s donation. These cells undergo a rigorous screening process to ensure that they are safe. These cells also have some of the greatest potential to treat illness and disease.
Several types of stem cells may be used in research. These include:
- Adult stem cells: also sometimes referred to as “tissue stem cells”, these come from adult cells. They have a limited capacity to reproduce – they can only become more of the type of cell that they were harvested from. For example, a kidney stem cell can only become more kidney cells.
- Pluripotent stem cells: also sometimes referred to as “embryonic stem cells”, these cells come from donated embryos. These cells have the potential to grow into many other types of cells found in the human body and are therefore slightly more valuable to research than adult stem cells.
- Mesenchymal stem cells: this type of cell is adult or umbilical tissue. These cells can differentiate into different types of cells and are generally found in bone marrow, fat tissue, and donated umbilical cords.
- Induced pluripotent stem cells (iPSC): this type of stem cell is an adult stem cell that has been manipulated genetically to mimic pluripotent stem cells. They do have the ability to grow into most other cells in the human body but the process that turns them into iPSCs can be expensive and time-consuming.
Other terms that can be used to describe stem cells include “allogeneic” and “autologous”. Allogeneic refers to stem cells that were donated by someone and autologous refers to cells that come from the patient’s own body.
Potential Treatments Using Stem Cells
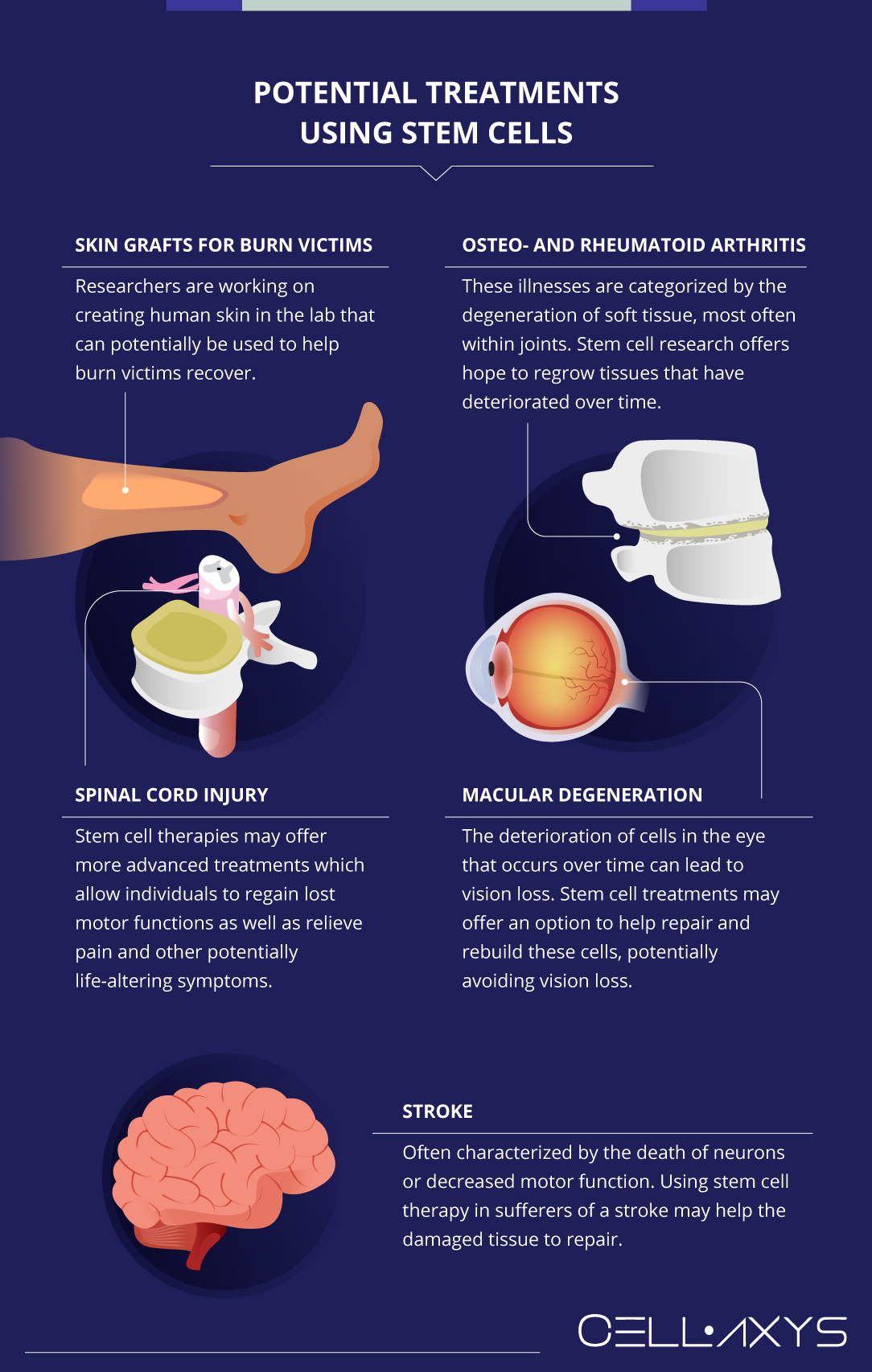
Due to the vast potential of these cells, there are research studies and clinical trials for almost any ailment and all body parts. Some of the most promising research includes:
- Skin grafts for burn victims: researchers are working on creating human skin in the lab that can potentially be used to help burn victims recover.
- Osteo- and rheumatoid arthritis: these illnesses are categorized by the degeneration of soft tissue, most often within joints. Stem cell research offers hope to regrow tissues that have deteriorated over time.
- Spinal cord injury: people who have been in accidents or have otherwise experienced trauma to the spinal cord may have new hope for treatment. Treatment for injury to the spinal cord has mostly involved managing the symptoms and learning how to live with them, rather than treating them. Cell-based therapies may offer more advanced treatments which allow individuals to regain lost motor functions as well as relieve pain and other potentially life-altering symptoms.
- Macular degeneration: the deterioration of cells in the eye that occurs over time can lead to vision loss. Cell-based treatments may offer an option to help repair and rebuild these cells, potentially avoiding vision loss.
- Stroke: often characterized by the death of neurons or decreased motor function. Using cell-based therapies in sufferers of a stroke may help the damaged tissue to repair.
Scientific Research vs. Clinical Trials
The process of designing a research proposal is extensive and, in some cases, can take years. Scientists could have an idea of what they want to study but they still have to secure funding, acquire the materials, find participants (human or animal), and be approved. A study cannot become a clinical trial unless the research shows it may be a viable option.
This process begins with a proposal, which must outline the specific steps that will be taken to conduct the research. The proposal must be as informative as possible and outline all of the possibilities that could occur.
For research to move forward in the process, it does not need to be approved by the FDA. Many research studies occur at universities and private companies. The approval process for these studies is typically kept in-house and involves employees of the institution, not necessarily anyone affiliated with the government.
Research studies can take anywhere from months to years, and even decades. Once the research process is completed, scientists can move on to clinical trials.
Clinical trials are what occurs when a new drug or treatment has been researched extensively and doctors are ready to try it on people outside of a lab setting. Similar to research studies, these undergo rigorous guidelines and screening processes. Clinical trials are often small, having on average less than 200 participants.
Most clinical trials involve a “control group”, which is a group that receives a placebo treatment. The purpose of having a control group is to determine how effective the treatment is compared to no treatment at all.
Clinical trials are a crucial aspect of finding treatments for almost every illness. Every drug on the market has been researched and undergone clinical trials.
Clinical Trials For Stem Cells
Title: “Mesenchymal Stem Cell Augmentation in Patients Undergoing Arthroscopic Rotator Cuff Repair”
Company: Rush University Medical Center
This study has 100 participants and seeks to measure the benefit of cell-based treatments. The research is based on rotator cuff injuries and the repair procedure for them. Some of the participants received cell-based treatments in the shoulder, while others did not.
The results are predicted to be that patients who have received cell-based treatments will have a generally better recovery than those who did not. The study seeks to measure recovery progress by measuring the range of motion, strength, function, and overall tissue health.
At the end of the trial, doctors will use imaging techniques that capture soft tissue (such as MRI or ultrasound) to measure tissue repair in the participants. The goal of this study is to eventually enhance treatment options available for rotator cuff injury.
Company: Mayo Clinic
This small study has only 10 participants. It seeks to observe the recovery process of people who have suffered a traumatic spinal cord injury, such as from a car accident or a fall. Using autologous adipose stem cells, doctors will inject these into a patient’s injury site and monitor their progress for 96 weeks.
Doctors will be monitoring changes in patients’ bloodwork as the study progresses. The expected outcome is that patients receiving this treatment will have a more successful recovery than those who do not receive cell-based treatments.
Company: Qilu Hospital of Shandong University
Containing only 8 participants, this study seeks to monitor the progression of early knee arthritis and how it may be impacted by injections of autologous mesenchymal stem cells. Over 6 months, doctors will monitor patients’ soft tissue. Arthritis progresses at a certain rate, and doctors will be monitoring the rate of progression after receiving stem cell injections.
Company: Stanford University
Using a surgical intervention technique, doctors will perform a randomized mesenchymal stem cell infusion on 20 patients. Some will receive the treatment, and others will not – patients do not know if they have received the cell-based treatment or surgery alone.
The purpose of this study is to monitor how stem cell treatments can impact cartilage repair and soft tissue structure in patients with a certain type of osteoarthritis.
Company: ReNeuron Limited
110 participants will undergo surgery. All of the patients will have had an ischemic stroke before the clinical trial. Half of the patients will receive stem cell injections, and half will not. The study will monitor the progress of each patient using tests that measure their ability to perform certain tasks, and range of motion.
After 12 weeks, patients who received stem cell injections will be compared to patients who received a placebo. The prediction is that patients who received cell-based treatment have improved motor skills compared to those who received a placebo treatment.
Each of these trials is performed under rigorous stipulations. They provide potential treatments for a myriad of conditions. These are only 5 examples – there are thousands of similar clinical trials occurring right now.
The Future of Regenerative Medicine
Each clinical trial and the research studies that preclude them provide patients with new hope for their untreated illnesses. It is important that such studies are closely monitored by experts and performed correctly to ensure accurate results.
Once a treatment reaches a clinical trial, it is relatively close to becoming available to patients all around the world. The future of regenerative medicine depends on trials such as these to advance treatment options.
Companies such as CELLAXYS offer autologous cell-based treatments to patients who are suffering from such illnesses or ailments. Focusing on injury or degenerative disease, CELLAXYS seeks to improve patients’ quality of life by administering cell-based therapies.
Sources
Footnotes
- Weiss JN. Mesenchymal Stem Cell Augmentation in Patients Undergoing Arthroscopic Rotator Cuff Repair. Orthopedic Stem Cell Surgery 2021 (pp. 3-6). Cham: Springer International Publishing.
- Weiss JN. Treatment of Early Knee Osteoarthritis with Autologous Adipose-Derived Mesenchymal Stem Cells. Orthopedic Stem Cell Surgery 2021 (pp. 135-140). Cham: Springer International Publishing.
- Kalladka D, Sinden J, Pollock K, Haig C, McLean J, Smith W, McConnachie A, Santosh C, Bath PM, Dunn L, Muir KW. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): a phase 1, first-in-man study. The Lancet. 2016;388(10046):787-96.
References
- Complete human skin grown in lab. European Commission. Accessed 9/17/2023.
- Use of MRI Labeling Technique to Track Mesenchymal Stem Cell Survival in Orthopaedic Conditions. U.S. National Library of Medicine. Accessed 9/17/2023.
- Adipose Stem Cells for Traumatic Spinal Cord Injury (CELLTOP). U.S. National Library of Medicine. Accessed 9/17/2023.
CELLAXYS does not offer Stem Cell Therapy as a cure for any medical condition. No statements or treatments presented by Cellaxys have been evaluated or approved by the Food and Drug Administration (FDA). This site contains no medical advice. All statements and opinions are provided for educational and informational purposes only.
Dr Pouya Mohajer
Author
Pouya Mohajer, M.D. is the Director of Spine and Interventional Medicine for CELLAXYS: Age, Regenerative, and Interventional Medicine Centers. He has over 20 years of experience in pain management, perioperative medicine, and anesthesiology. Dr. Mohajer founded and is the Medical Director of Southern Nevada Pain Specialists and PRIMMED Clinics. He has dedicated his career to surgical innovation and scientific advancement. More about the doctor on this page.
Dr Pejman Bady
Contributor
Dr. Pejman Bady began his career over 20 years ago in Family/Emergency Medicine, working in fast-paced emergency departments in Nevada and Kansas. He has served the people of Las Vegas as a physician for over two decades. Throughout this time, he has been met with much acclaim and is now the head of Emergency Medical Services in Nye County, Nevada. More about the doctor on this page.